CBS News Live
CBS News Detroit: Local News, Weather & More
Watch CBS News

Prosecutors charged Detroit Police Officer Caleb Williams, who is accused of threatening his nephew with a taser.

Jorrel Tyler Jackson, 31, of Seffner, Florida, is sentenced to six and a half years in prison in connection with an international fraud conspiracy that targeted seniors in multiple states, including Michigan, the U.S. Attorney's Office announced.

Peter Meijer had joined the race in November 2023.

The National Bobblehead Hall of Fame and Museum on Friday unveiled the new limited edition bobblehead featuring Oakland University basketball player Jack Golhke.

The driver was arrested after police said she had a blood alcohol content of .17%.
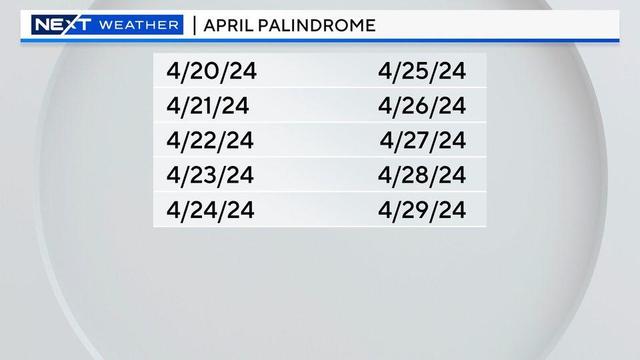
This week has been full of palindromes. Meaning the dates are read the same forwards and backward.

Noor Kestou was taken into custody by Macomb County detectives on Wednesday, April 24.

Downtown Detroit is booming with NFL fans, thanks to Day 1 of the draft. The influx is also a boost for area businesses.

The first day of the 2024 NFL Draft in Detroit did not disappoint. From the crowds to athletes and celebrities attending, Thursday also ended with hitting a record.

The Detroit Lions selected Alabama cornerback Terrion Arnold on Thursday in the first round of the 2024 NFL Draft.

NFL Commissioner Roger Goodell announced that more than 275,000 fans attended the first day on Thursday, according to a spokesperson.

Michigan football quarterback J.J. McCarthy is starting his NFL career with the Minnesota Vikings after being selected No. 10 in the first round of the 2024 NFL Draft.

To promote sustainable farming, members of the NFL and officials from the White House took part in a ribbon cutting on Thursday for a GroShed in a Detroit community.

The NFL Draft announced it is halting entry into Thursday's event after reaching maximum capacity in downtown Detroit.

Prosecutors say Steven Wheeler, 30, is accused of fatally shooting his wife during an altercation in October 2023.

Fire officials are investigating after a man was injured in an Ann Arbor house explosion Monday morning. CBS News Detroit's Jordan Burrows gives the latest updates from the scene.

The Michigan State Spartans celebrated a weekend of commencement ceremonies.

Families poured in to check out this Michigan treasure.

The City of Detroit's Easter Fun Fest returns to provide families an opportunity to not only get outside and be active, but ring in the holiday with some fun.

Mixed media artist Donald Calloway has been creating art in some form or fashion most of his life. His art studio takes you on a journey, as his creative collection of his art runs the gamut.

Soon after a positive test, Dorfman found himself hospitalized, on a ventilator and in a medically induced coma.
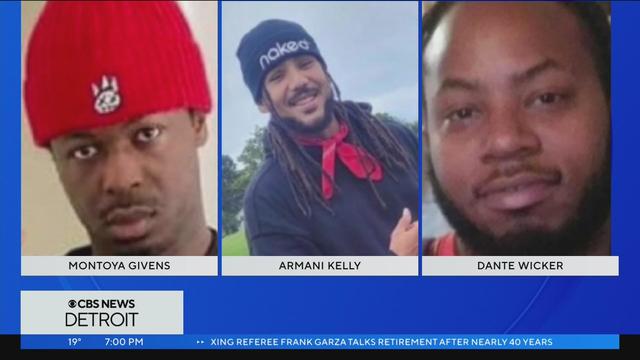
It has been 12 days since anyone has seen Armani Kelly, Montoya Givens and Dante Wicker. Now, a Facebook live video conversation has surfaced where Kelly is seen talking with three men about coming to Detroit only two days before he went missing.

A Plymouth man's cancer diagnosis hit the reset button on how he lived his life. Since then, he and his family have been giving back to others facing the same struggles.

The Sphinx organization gives a platform to Black and Latinx performers of all ages.